Teaching Assignments at UH. Robert Comito teaches two recurring classes in the Chemistry Department at UH.
Chem 2325: Fundamentals of Organic Chemistry. (formerly Chem 3332) This is the second semester of UH's undergraduate course sequence on organic chemistry. Students learn to recognize fundamental bond-forming reactions and functional groups, and then to apply them to organic synthesis. In that context, students acquire analytical techniques and bonding concepts and apply them to predicting and explaining the outcomes of organic reactions. This course surveys the major functional groups of organic chemistry, using spectroscopy, bonding, and arrow-pushing diagrams as a basis for explaining how reactions work and why. Specific functional groups include ethers, polyenes, aromatics, carbonyls, and amines. Specific techniques include UV/Vis spectroscopy and 13C-NMR. Within this framework, students are expected to interpret spectroscopic data, to propose reasonable syntheses of organic molecules, and to explain reaction outcomes in terms of bonding and electron-pushing formalisms. I assume a basic mastery of Organic Chemistry I, including Kekulé structures, the nomenclature of organic compounds, 1H-NMR, IR spectroscopy, and arrow-pushing mechanisms.
Course materials: Organic Chemistry, Wade, L. G. 8th edition. Pearson: 2013.
Semesters taught: Spring 2020, 2021, and 2023
Chem 6311: Mechanisms. This is a first-year chemistry graduate course designed to teach students how to evaluate organic reaction mechanisms within empirical and hypothesis-driven research. Students learn to propose plausible mechanisms for organic reactions, to design experiments that evaluate mechanistic hypotheses, and to communicate mechanistic arguments. The course first provides a pedagogical framework for proposing reasonable reaction mechanisms in organic chemistry, by reviewing key paradigms of bonding and reactivity and examining several classes of reactions and reactive intermediates of general importance. Then, toward the goal of testing reaction mechanisms experimentally, we cover kinetics, isotope effects, and other experimental approaches. Finally we cover physical concepts of strain and conformational analysis as they relate to organic reactivity.
Course materials: (A) Writing Reaction Mechanisms in Organic Chemistry, 3d edition. Savin, K. A. Elsevier: 2014. (B) Modern Physical Organic Chemistry, Anslyn, E. and Dougherty, D. University Science Books: 2006. (C) Course notes and assigned primary literature.
Semesters taught: Fall 2018, 2019, 2020, 2021, and 2022
Teaching Innovations
Drag-and-Drop Quizzing:
I teach both of the classes above using active learning and scaffolding. Pedagogical research continues to show that active learning improves learning gain and inclusivity, compared to a pure lecture instructional format. Active learning mitigates classroom inequalities by promoting full participation and by giving the instructor real-time feedback. Active learning engages problem solving and critical thinking skills that passive notetaking does not. Teaching problem solving and critical thinking further benefits from effective instructional scaffolding, in which structure guides students through complex tasks. Unfortunately, active learning and scaffolding are less commonly employed in chemistry courses than in any other STEM subject. This disparity reflects the fact that organic and general chemistry are typically large service classes in which paper-and-pencil activities are less scalable than digital ones. Many chemistry problem types, especially bonding, synthesis, and mechanism questions are inherently graphical, and lose much of their conceptual richness when adapted to multiple-choice or short-answer formats.
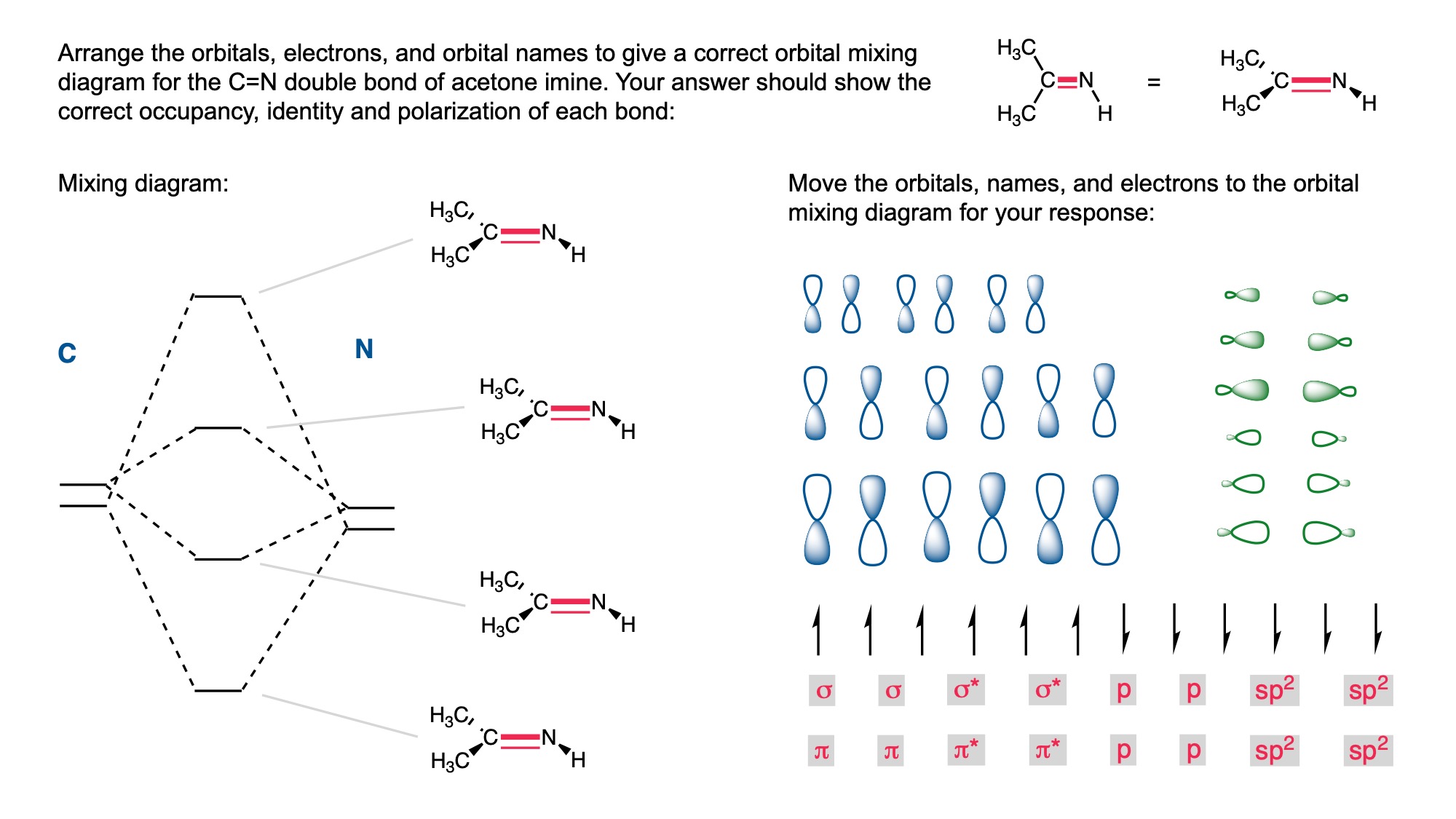
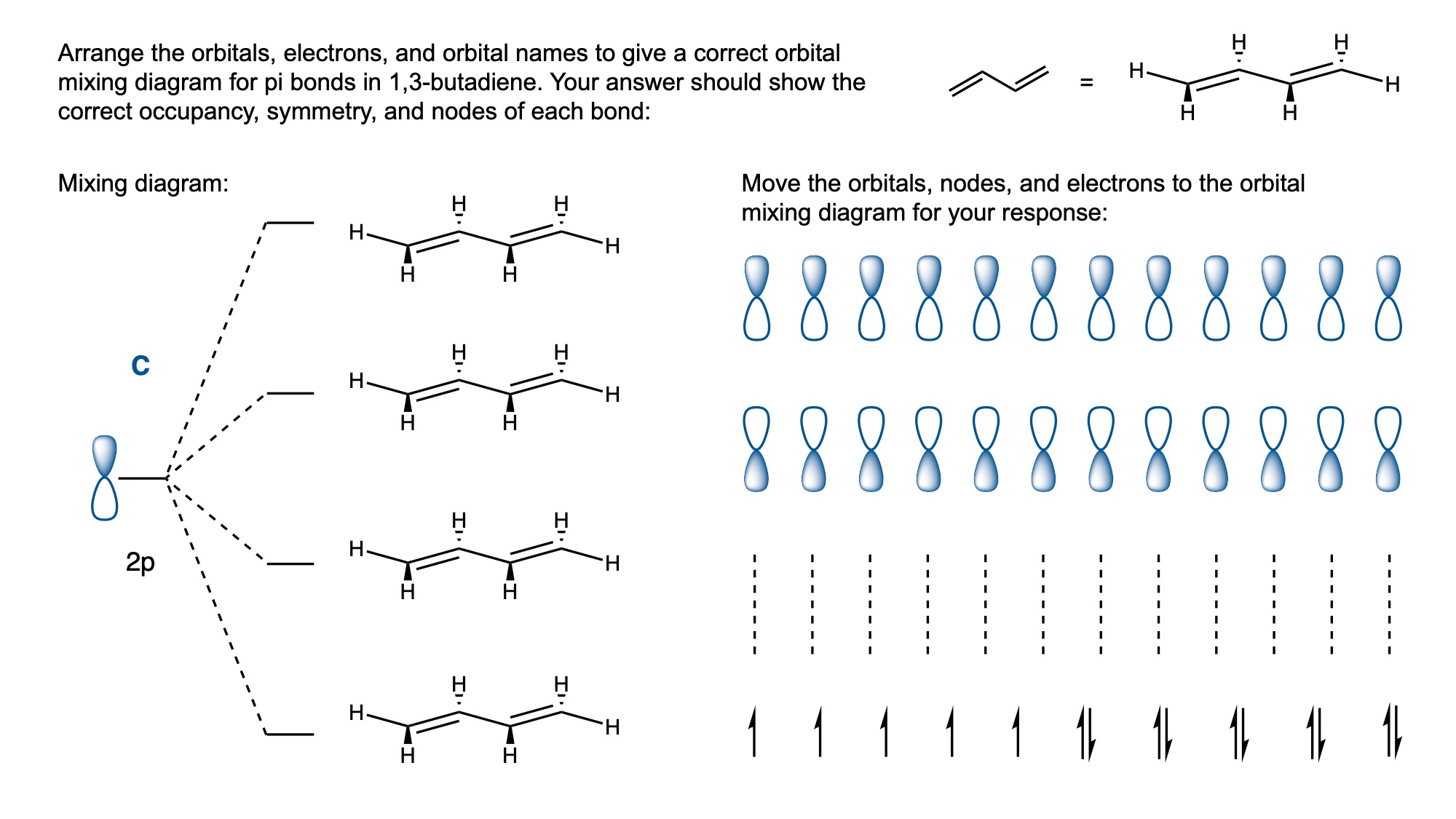
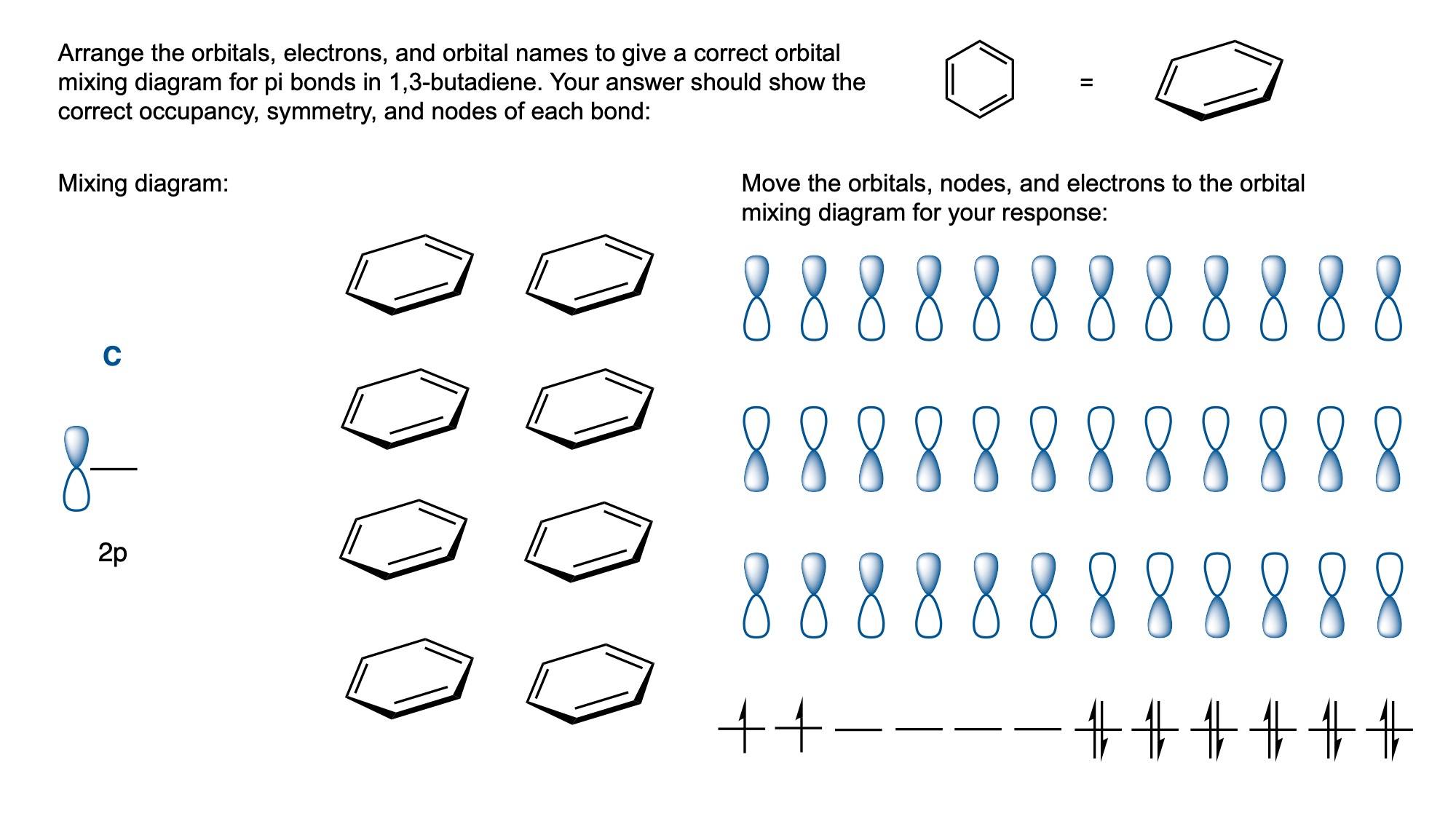
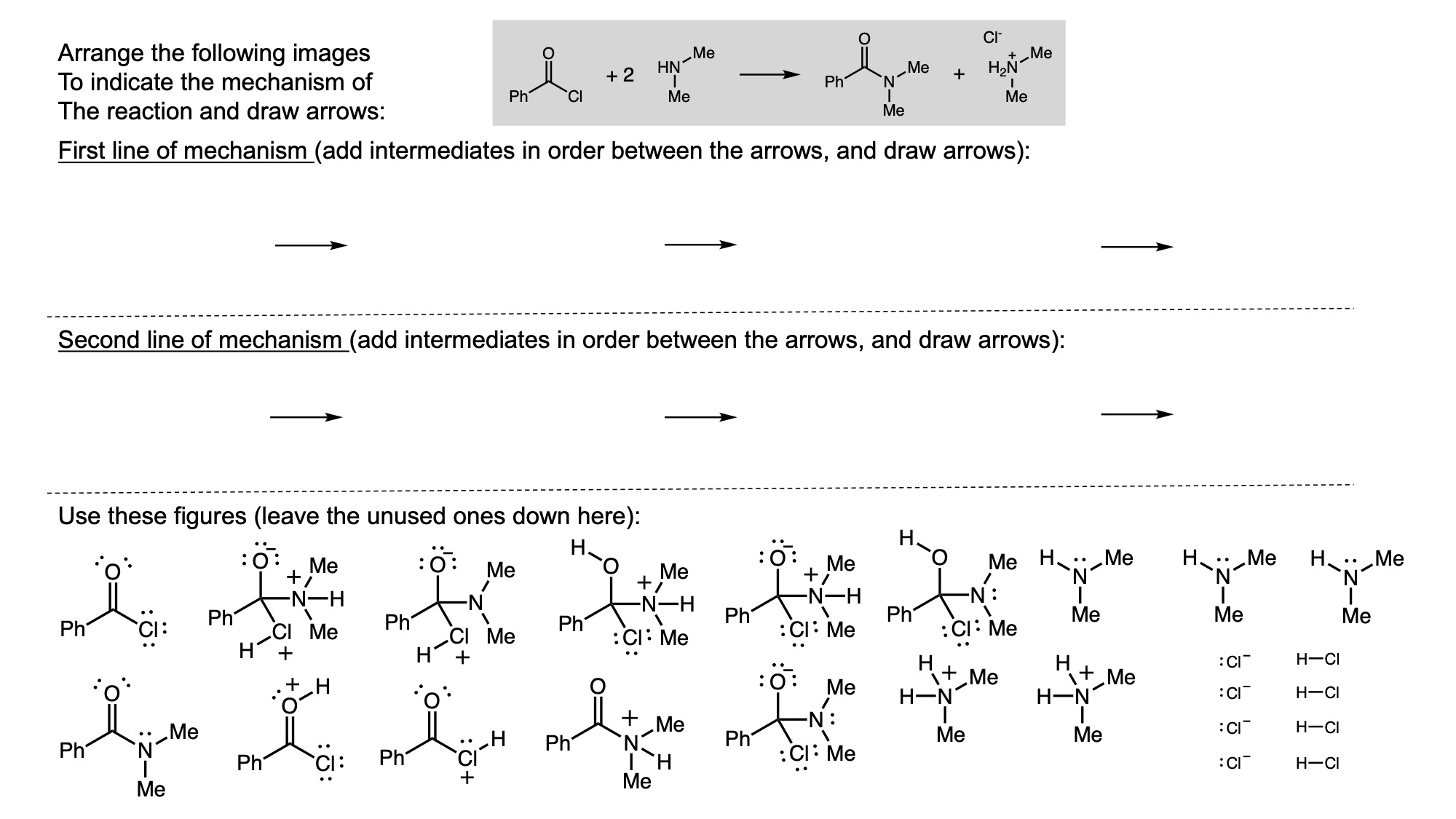
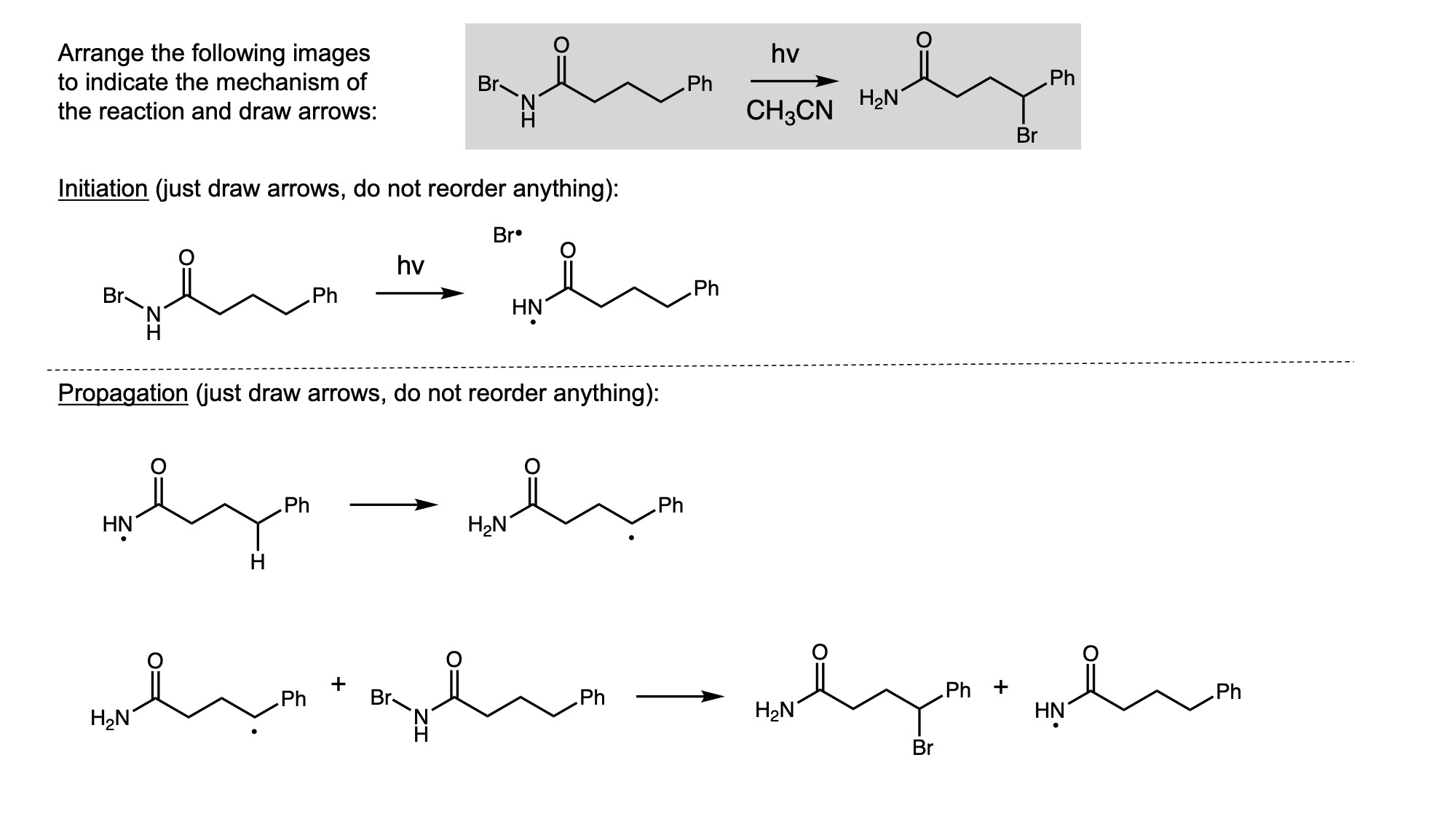
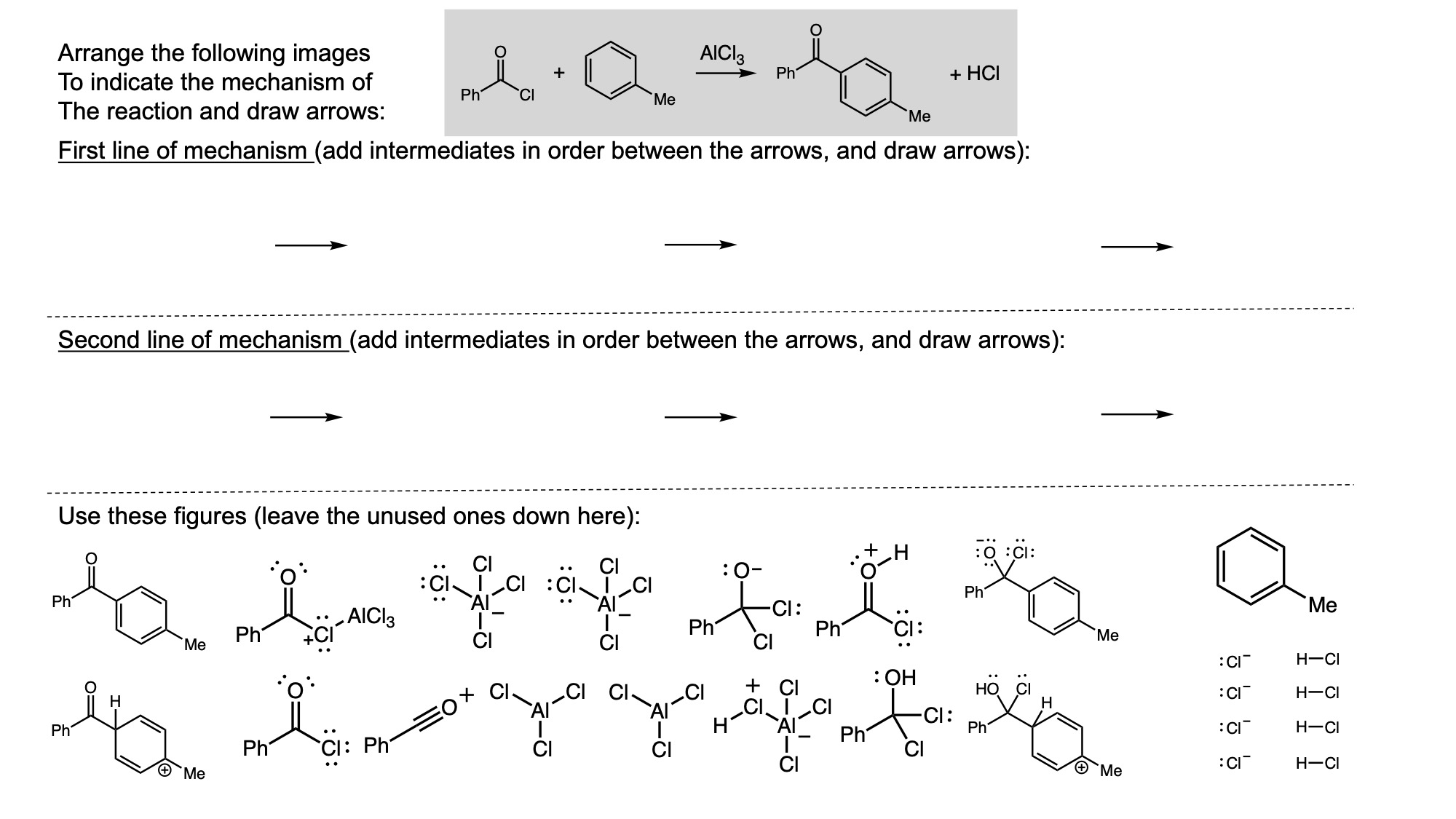
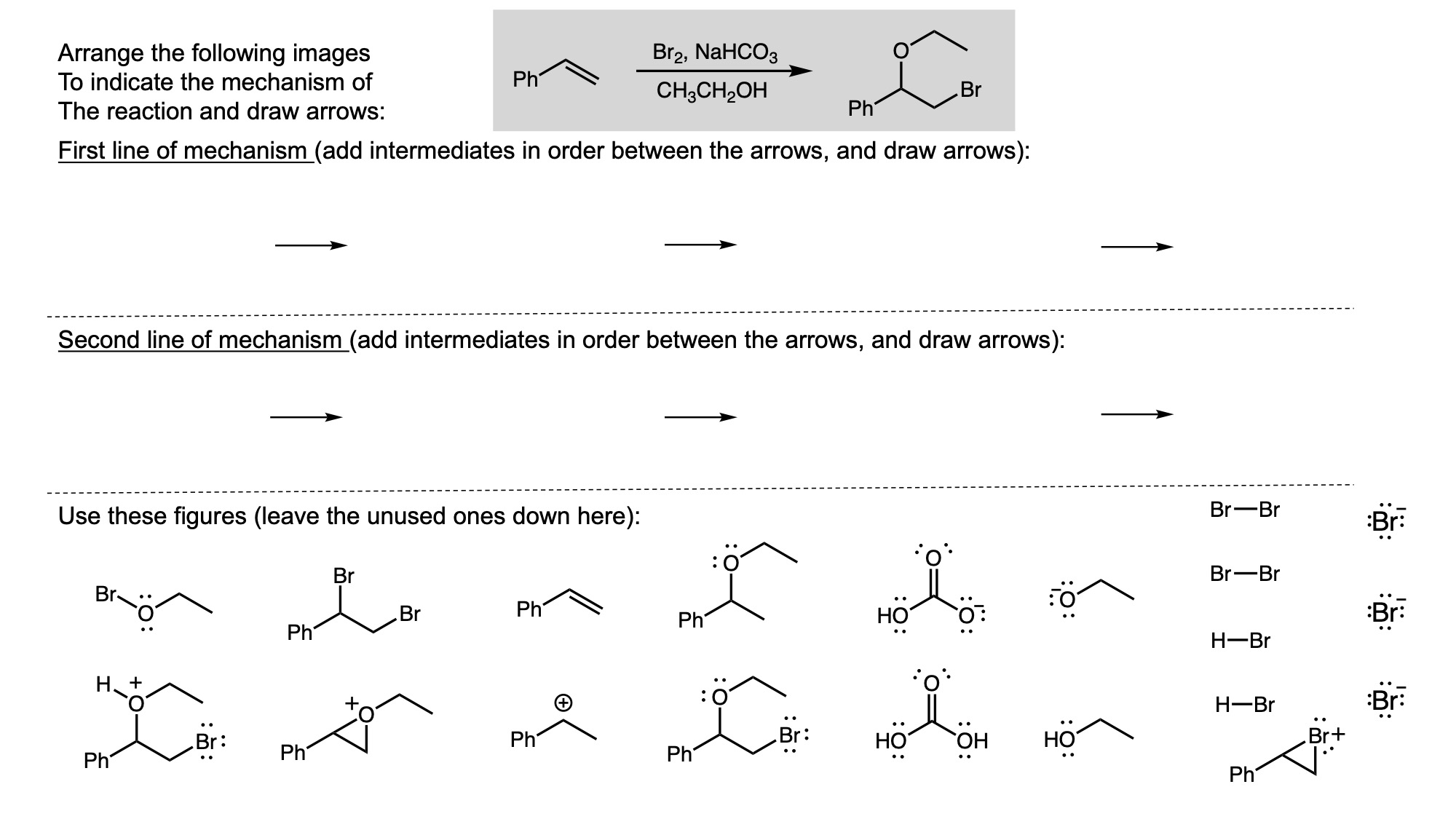
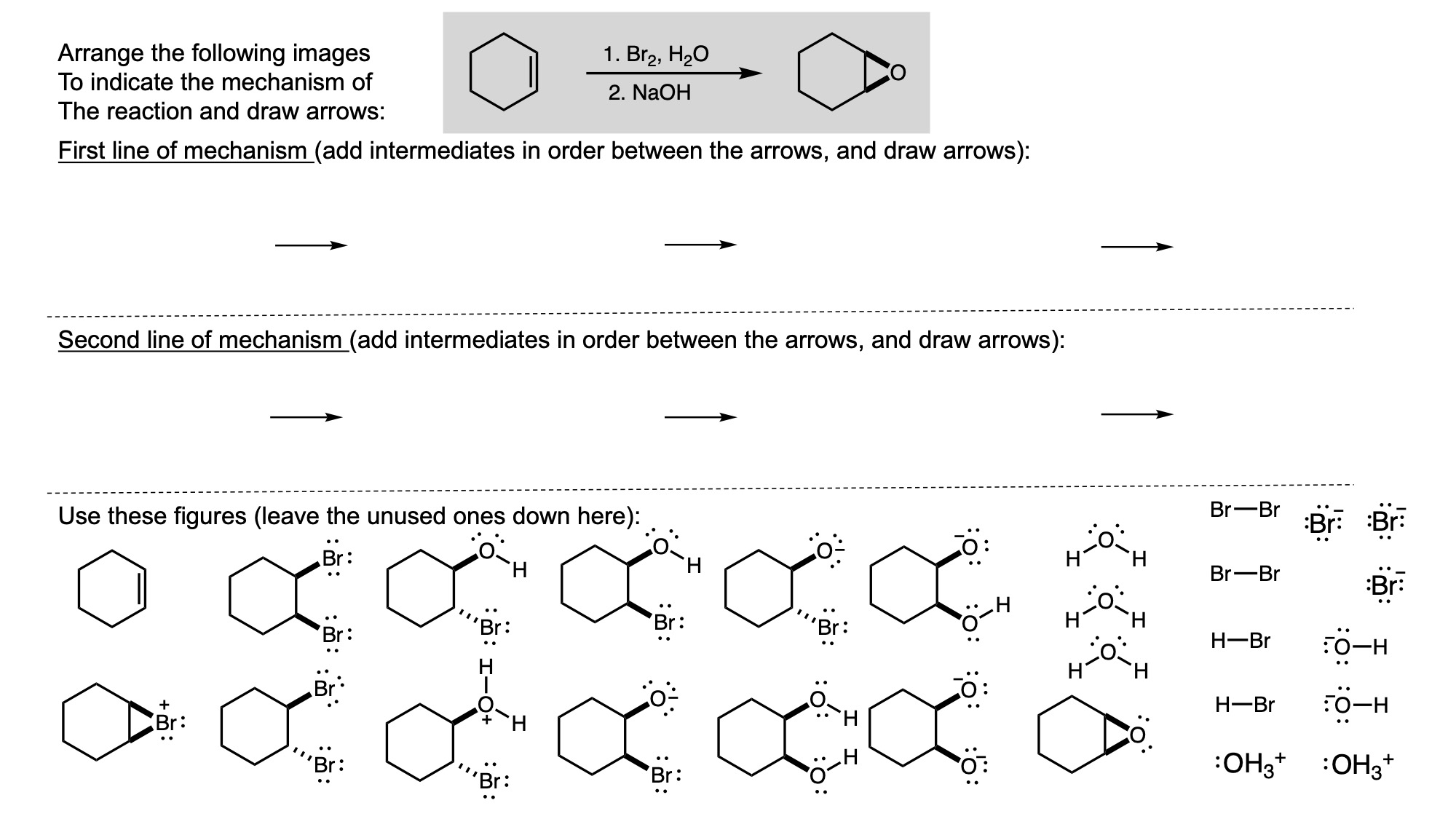
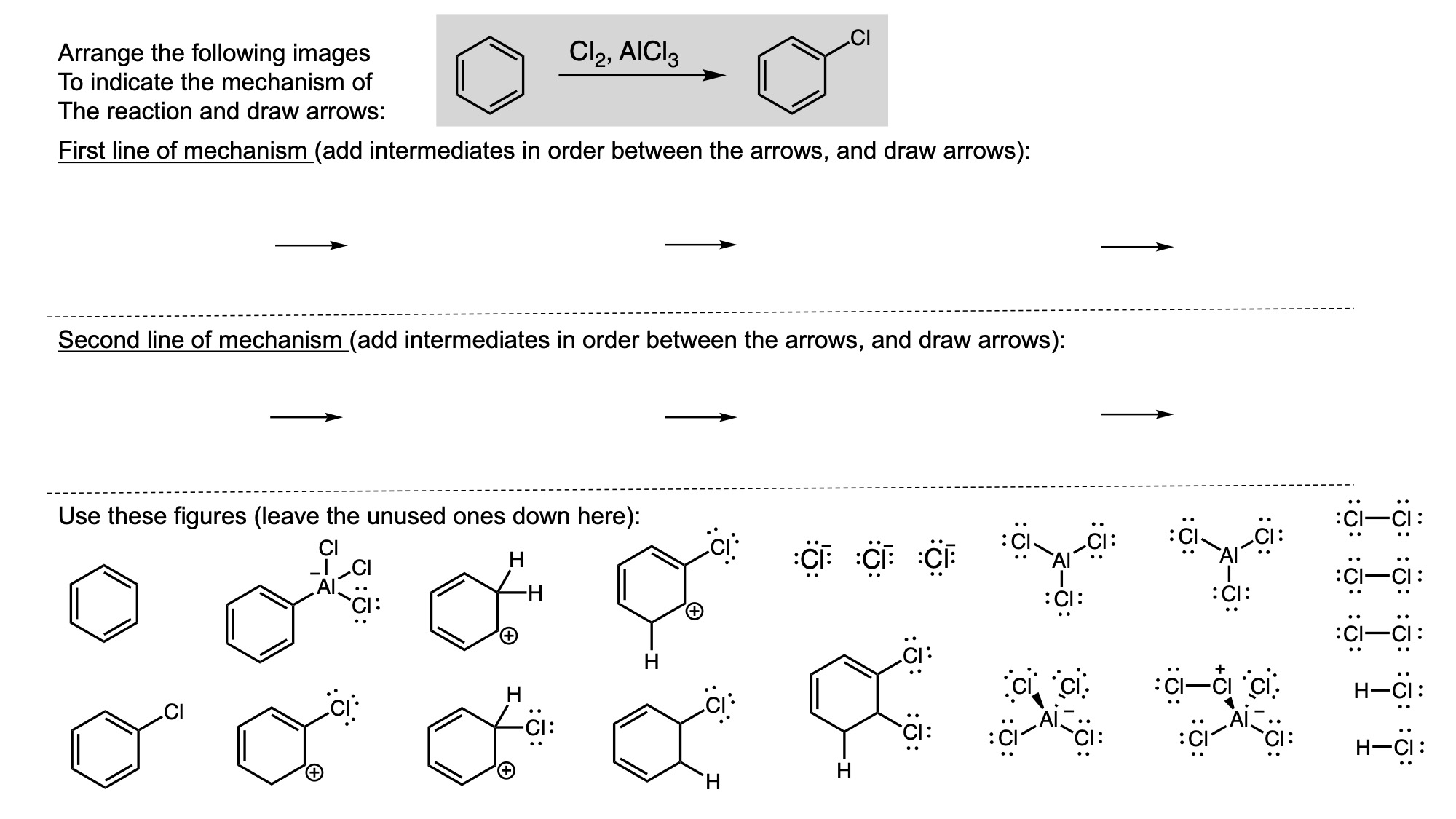
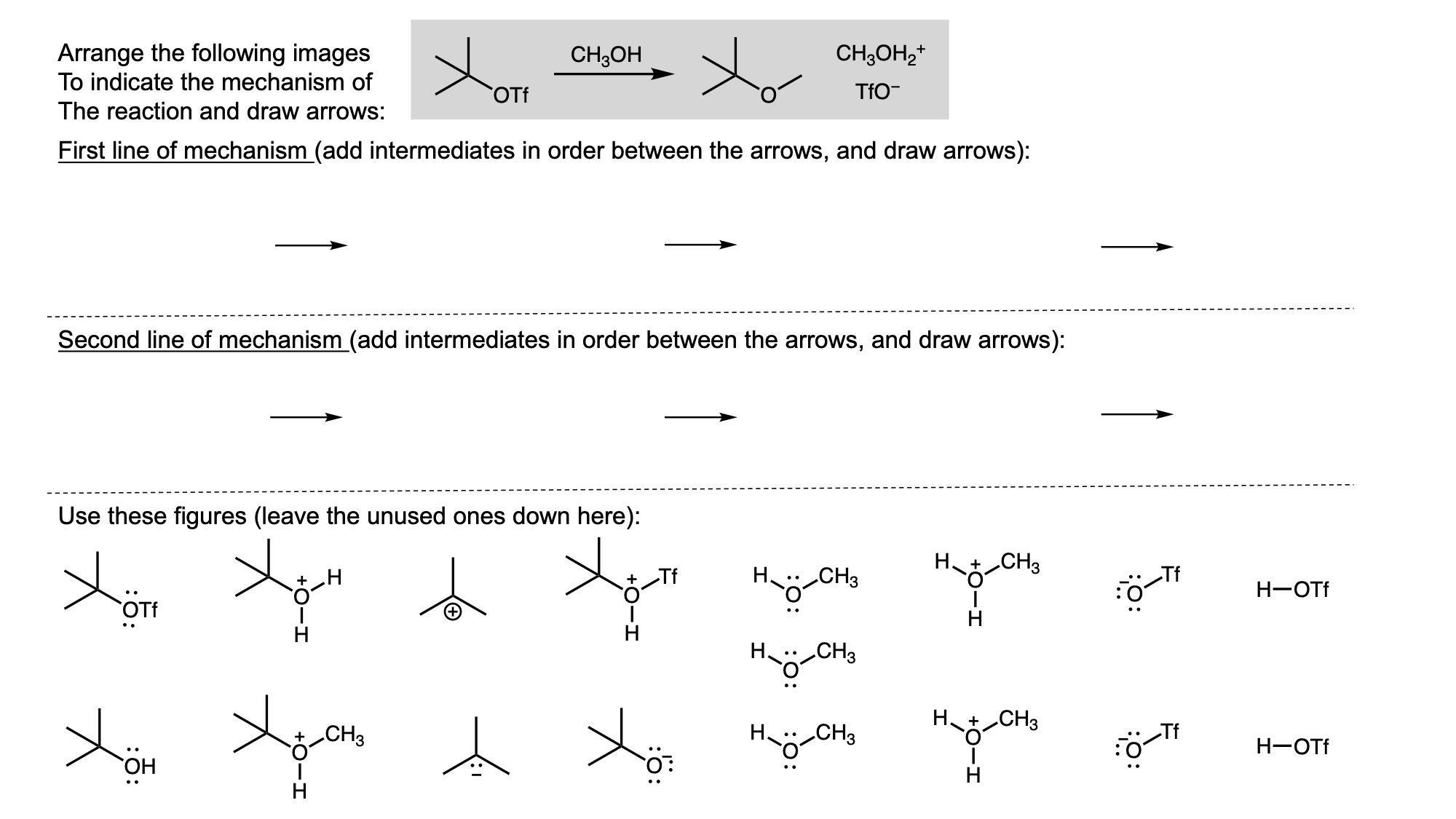
In order to digitally implement these kinds of questions, I have been developing them as drag-and-drop (DD) questions. This effort has been supported by a UH grant through the Teaching Innovation Program. I have also used DD questioning extensively during the COVID-19 pandemic (2020-2021), when remote digital activities were the only option. The figure below illustrates my approach using two quizzes from Chem 6311. Problem prompts are typically posed to students on the projector, while an editable artboard with movable images is distributed to the class. To generate a response, students are directed to 1) rearrange the images on the artboard, 2) output a response image, and 3) upload their responses. In the examples below, I generated movable images in ChemDraw and used Microsoft PowerPoint as the artboard. The artboards were distributed and responses were collected digitally using Blackboard. Students were also given the option to submit freehand responses on paper. Responses were then graded digitally and anonymous sample answers were raised on the projector for discussion.
Mechanism problems include multiple options for intermediates, drawn out as full Lewis structures, and reaction arrows to structure the answer. Students can move the structures and draw curved arrows to generate a response. Bonding questions involve skeletal orbital mixing diagrams with movable orbitals, orbital labels, and electron occupancy arrows. Students can rearrange the orbitals, labels, and arrows to generate a response. Synthesis questions work similarly, with movable reaction conditions and intermediates. Alternatively, text boxes are used for short free-response components, for example proposing reagents for synthesis questions. Certain graphical elements are fixed in place to format the responses, such as straight reaction arrows used in synthesis and mechanism questions.
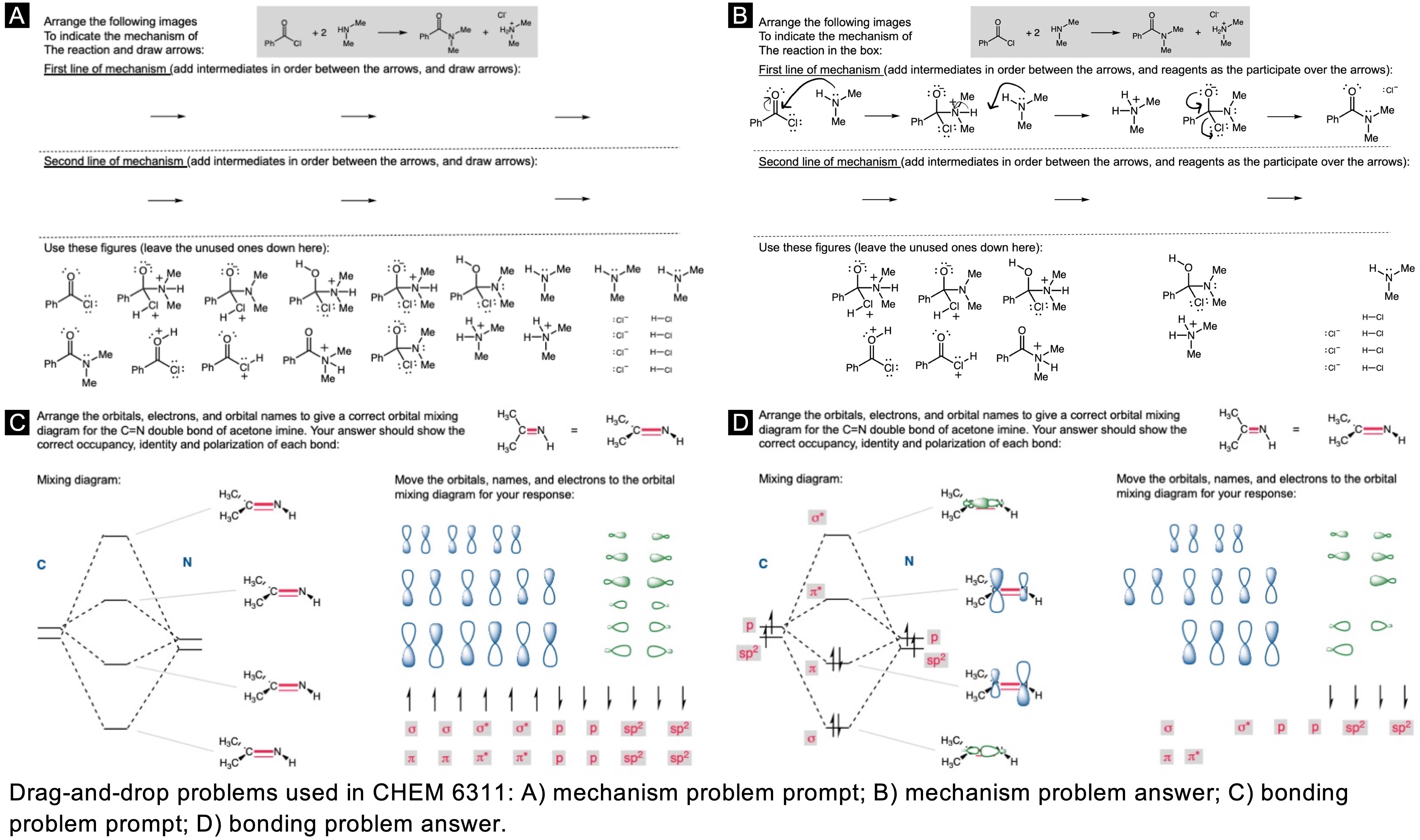
Sample Problems. I encourage broader adoption of DD questions and have provided my own sample problems. Please click on any of the images below to download a problem artboard as a .pptx file.
Control Experiments in Chemistry:
Control experiments examine the causal role of single variables in a complex system. They are heavily used in mechanistic chemistry because the chemist controls which components are added to a reaction. Moreover, control experiments represent the simplest examples of hypothesis testing, and thus represent a great way to teach experimental design and critical thinking. Control experiments are formally taught in other scientific disciplines, and most students are familiar with the concept in the context of clinical studies of medicines. However, control experiments are rarely covered in chemistry and there are few teaching materials to build on. To address this disparity, I have been teaching Chem 6311 with a lesson on control experiments. I organize common control experiments into five categories:
Omission Experiments: In an omission experiment, the chemical reaction under study is repeated in every detail except that the component being analyzed is left out. That component can be a substance (chemical reagent, catalyst, or solvent) or it can be a feature of the reaction conditions or setup (heating, UV irradiation, pressure, or reactor design). The reaction that occurs in the absence of that component is often called a background reaction. The basic goal of an omission experiment is to determine whether a component serves as a spectator or a participant in a reaction.
Scavenging Experiments: Like an omission experiment, in a scavenging experiment the chemical reaction under study is repeated in every detail except that the component being analyzed is actively removed, sequestered, or quenched during the reaction. Usually a scavenging (or quenching) agent is added to the reaction to accomplish this. Typically, the component being studied is an intermediate or byproduct that cannot simply be left out. Scavenging experiments make two key assumptions: 1) that the scavenging agent successfully reacts with the component being studied, and 2) that the scavenging agent does not interfere with the reaction in any other way. Sometimes, these assumptions also need to be tested with additional control experiments, especially validity studies. But usually, a few widely accepted scavengers are used in very specific contexts. For example, molecular sieves are common scavengers for water. Radical traps are common scavengers for organic radicals. The mercury drop test is likewise commonly used to scavenge metal nanoparticles.
Replacement Experiments: In a replacement experiment, the chemical reaction under study is repeated in every detail except that the component being studied is swapped for something else. The goal of a replacement experiment is often to evaluate whether the component is serving a trivial role. If the replacement experiment gives a similar result to the reaction being studied, then that outcome would be evidence for the swapped out substance serving as a source of the swapped in substance during the reaction. For example, if a presumed catalyst is actually serving as a source of a Bronsted acid, then replacing the presumed catalyst with that Bronsted acid should result in a successful reaction.
Resubmission Experiments: In a resubmission experiment, the chemical reaction under study is repeated in every detail except that the a presumed intermediate or product is added at the beginning of a reaction. The resubmitted material should be labeled in such a way that its fate can be distinguished from the product of the reaction. The goal of a product resubmission experiment is to determine whether the product once formed is stable and whether it influences the reaction. For example, if the percent recovery of the resubmitted product is low or if it deteriorates in any way (e.g. loss of stereochemistry) then the product is not stable under the reaction conditions. Likewise, if the reaction yield or rate decreases in the resubmission experiment, then product inhibition is likely occurring. By contrast, the goal of an intermediate resubmission experiment is usually to determine whether the resubmitted substance is a plausible intermediate, in which case it would be converted to the expected product. However, if the resubmitted material is recovered, converted to something else, or if the reaction yield decreases, then it is probably not an intermediate.
Validity Experiments are reactions in which the component being studied is submitted to a chemical reaction with a known mechanism or outcome. Trivially, validity experiments are used to determine whether a substance is pure or an apparatus is in working condition. In that case a known reaction is performed to determine whether a reported yield can be reproduced. However, validity experiments can be used to confirm that a component serves a particular role in a reaction or control experiment. For example, if a substance serves as a radical trap, then it should suppress a reaction with a known radical mechanism.
The lesson can be downloaded here in full. We encourage interested professors to use these notes in their own class. Additionally, I would gladly accept feedback or examples of control experiments by email.